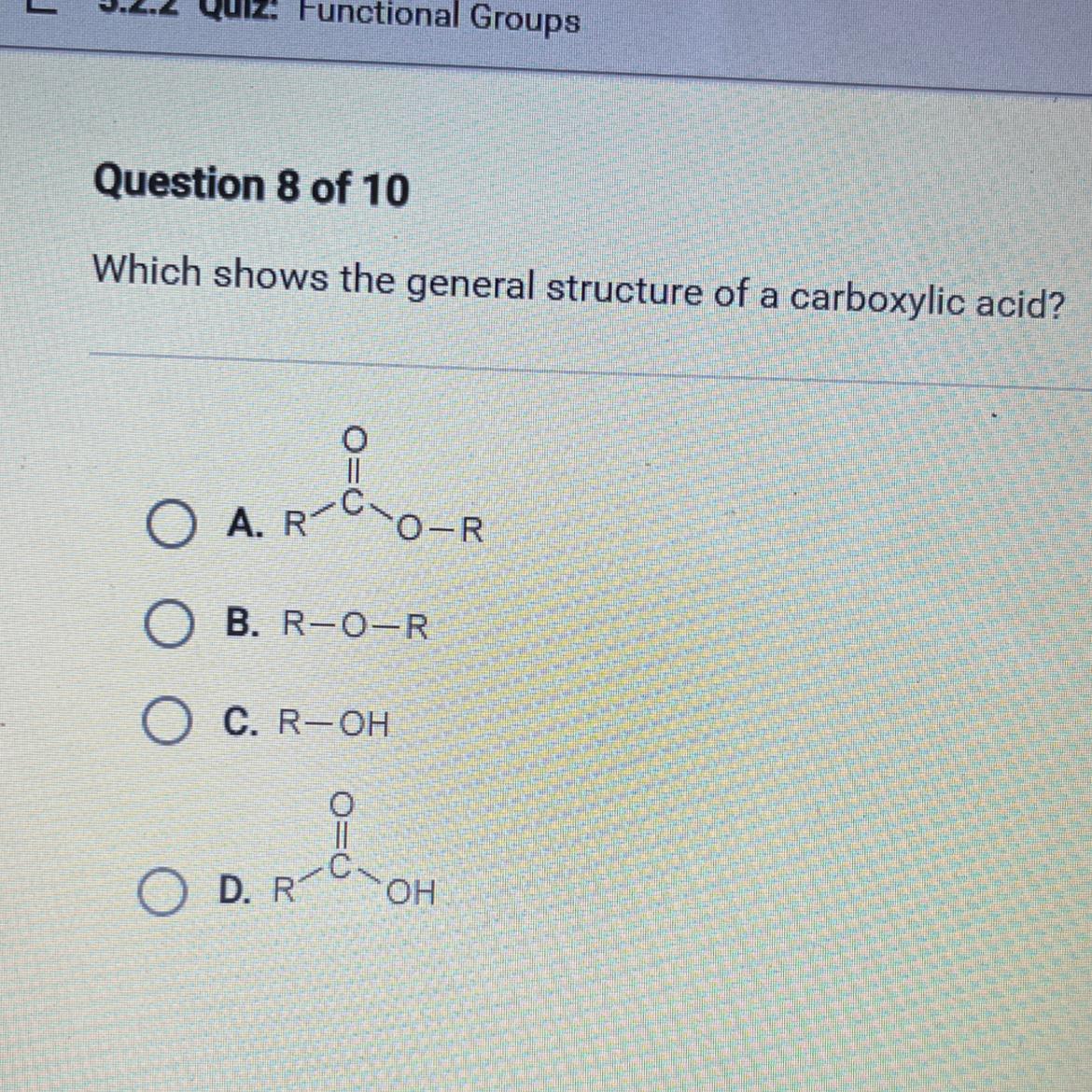
Which shows the general structure of a carboxylic acid?
O A. R
O-R
R
O R
B. R-O-R
C. R-OH
OD ROH
Answers
The general structure of a carboxylic acid is shown by option D.
In organic chemistry, a carboxylic acid is an organic acid that consists of a carboxyl group connected to an R-group. the overall components of carboxylic acid are R−COOH or R−CO₂H, with R referring to the alkyl, alkenyl, aryl, or other institution. Carboxylic acids arise broadly.
A carboxylic acid is a natural compound that incorporates a carboxyl group (C(=O)OH). the general formulation of a carboxylic acid is R–COOH, with R referring to the relaxation of the molecule. A carboxylic acid may be the concept of a mixture between functional corporations: an alcohol institution, related to hydrogen certain to oxygen, which attaches to a carbonyl institution, involving a carbon double sure to oxygen.
Learn more about carboxylic acid here:-https://brainly.com/question/26855500
#SPJ1
Related Questions
An unknown element sample has 2 isotopes present. The first isotope has a mass of 6.017 amu and is
7.30% abundant. The second has a mass of 7.018 amu and an abundance of 92.7%. Calculate the
average atomic mass of this element
Answers
Answer:
Calculating Atomic Mass
Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Explanation:
have a nice day
How are mitosis and meiosis similar? How are they different? Provide 1 way the two processes are the same and 2 ways the 2 processes are different.
Answers
Answer:
Similar: both processes of cell division; both processes take place in the nucleus of the cell
Different: mitosis divides into 2, meiosis divides into 4; one is the division of body cells and the other is of specifically sex cells
Explanation:
https://byjus.com/biology/mitosis-and-meiosis/
^ this has more info!
How can you improve your ability to see the indicator color change at the endpoint of a titration?.
Answers
Answer: Place a piece of white paper under the analyte flask throughout the titration
Explanation:
The end point of titration can be made easy by the use of indicators. But we have to be keenly observe for each drop from the burette because, the colour change will be sudden for a drop.
What is titration?Titration is an analytical technique used to determine the unknown concentration using a standard reagent of known concentration. The standard reagent of known concentration is called titrant and the one to be determined is called analyte.
Usually analyte is taken in a conical flask and titrant in a burette. The end point of titration is the point at which the reaction is completed in perfect stoichiometry.
In acid-base titrations, the indicators used having different colors in different pH. Hence, the indicator in the analyte will change in color when the acidity of the medium changes. We have to observe keenly for the color change because it happens for any drop.
The adding of titrant from the burette must be dropwise and thus we can get a time to notice the sudden colour change of indicator.
To get more about titration indicators, refer the link below:
https://brainly.com/question/27419598
#SPJ5
a
A sample of gas has a volume of 3.50 L and a pressure of
125 mm Hg. What will the new pressure be when the volume
is decreased to 2.75 L? The temperature stays the same.
Answers
Answer:
159.09 mmHgExplanation:
The new pressure can be found by using the formula for Boyle's law which is
[tex]P_2 = \frac{P_1V_1}{V_2} \\[/tex]
where
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
From the question we have
[tex]P_2 = \frac{125\times3.50 }{2.75} = \frac{437.5}{2.75} \\ = 159.090909...[/tex]
We have the final answer as
159.09 mmHgHope this helps you
What is oxidation state?
Answers
Answer:
a number assigned to an element in chemical combination which represents the number of electrons lost (or gained, if the number is negative), by an atom of that element in the compound
Explanation:
sodium, magnesium, iron is zero.
The definition of oxidation state is as follows: Oxidation state is a number given to the atom of an element when participating in a chemical reaction.
OXIDATION STATE:The atoms of elements are not always neutral i.e. carry no charge. They sometimes carry either positive or negative charges to become ions. Ions can either be cations (positively charged) or anions (negatively charged). The amount of charges are represented by numbers placed as a superscript in front of the element involved. These superscript charge assigned to a corresponding element during a chemical reaction is called oxidation state. The oxidation state of an element portrays the number of electrons gained or lost during the reaction.Therefore, oxidation state can be defined as a number given to the atom of an element when participating in a chemical reaction.
Learn more about oxidation state at: https://brainly.com/question/11313964
Solid calcium hydroxide is dissolved in water until the pH of the solution is 11.44. The hydroxide ion concentration [OH–] of the solution is:______.A. 1.1 * 10-11 M.B. 3.06 M.C. 8.7 * 10-4 M.D. 1.0 * 10-14 M.E. None of these.
Answers
Answer:
c the answer is c that is the answer
22) Calculate the energy of a photon of radiation with a frequency of 9.50 x 1013 Hz.
Answers
The energy of the photon with a frequency of 9.50×10¹³ Hz is 6.29×10¯²⁰ J
From the question given above, the following data were obtained:
Frequency (f) = 9.50×10¹³ Hz
Planck's constant (h) = 6.626×10¯³⁴ Js
Energy (E) =?The energy of the photon can be obtained as follow:
E = hf
E = 6.626×10¯³⁴ × 9.50×10¹³
E = 6.29×10¯²⁰ JTherefore, the energy of the photon is 6.29×10¯²⁰ J
Learn more: https://brainly.com/question/7957705
Draw the structure of the product that is formed when the compound shown below undergoes a reaction with 1 equivalent of ch3mgi and then is treated with water.
Answers
When the compound shown is treated CH3MgI followed by water, 1,3 - cyclohexadienol is formed.
Grignard reagent is an alkylmagnesium halide. Grignard reagents are important synthetic tools in chemistry. They are used to synthesize and number of compounds in the laboratory.
When the Grignard reagent, CH3MgI is reacted with compound shown followed by treatment with water, the product of the reaction is 1,3 - cyclohexadienol whose structure is shown in the second image attached to this answer.
Learn more: https://brainly.com/question/9309478
PLS HELP: Endocrine Organs Crossword
Answers
methane is called an organic compound why
Answers
Answer:
Explanation:
1) Organic compounds always contain only p-block elements (Groups III-VII), at least one of which must be carbon. 2) Organic compounds almost always contain one or more C-H bonds. ... Thus, all bonds are typically covalent in organic compounds. Methane (CH4) is the prototypical organic molecule.
Methane contains carbon, forming covalent bonds, and is found in living organisms, making it an organic compound.
Methane (CH₄) is considered an organic compound due to its molecular structure and occurrence in living organisms. Organic compounds are primarily composed of carbon atoms covalently bonded to hydrogen and often other elements like oxygen, nitrogen, sulfur, and more.
Methane consists of one carbon atom bonded to four hydrogen atoms through covalent bonds. Carbon's unique ability to form stable covalent bonds with other elements, including itself, leads to the vast diversity of organic molecules found in living organisms.
Methane is a crucial component of natural gas and is produced by various biological and geological processes. It is present in the digestive systems of animals, formed during decomposition, and plays a role in carbon and energy cycles.
Its prevalence in living systems and its molecular structure classify methane as an organic compound, reflecting the foundational principles of organic chemistry.
To learn more about Organic compounds here
https://brainly.com/question/28682641
#SPJ3
The complete question is :
Methane is called an organic compound why?
Add 2 teaspoons of baking soda and 2 teaspoons of citric acid to the foam cup. Add the half cup of water to the cup and stir. What is the temperature?
Answers
Answer:
Test for carbon dioxide gas.
The reaction between baking soda and citric acid will form sodium ions, citric acid ions, carbon dioxide gas, and water. So all you have to do is add the gas produced (by gas displacement method or something) and test whether the gas is carbon dioxide, with lime water or other methods like hydrogencarbonate indicator. Hope this helped!
Explanation:
Answer:
depends on amount, but the reaction's endothermic (meaning its cold, absorbs heat from surroundings)
I hope it helps.
Please help This is for my chemistry class and I need to get it done but I’m lost and need answers
Answers
Answer:
chlorine is a non metal.
sodium is a alkali metal.
helium is a noble gas.
nickel is a transition metal
Describe how the suspects used the chemicals found at the crime scene to make the copper coins look silver.
Answers
The chemicals found at the crime scene although not mentioned must be Sodium zincate.
Discussion:
An interesting demonstration to show plating and alloys is the conversion of copper coins to silver look-alike coins.
A 'copper' coin when dipped into a solution of sodium zincate in contact with zinc. The coin is plated with zinc and appears silver in colour.
This is a common chemistry classroom trick.
Read more on plating:
https://brainly.com/question/20078989
why does helium fusion require higher temperatures
Answers
Answer:
Nuclear fusion of hydrogen to form helium occurs naturally in the sun and other stars. It takes place only at extremely high temperatures. That's because a great deal of energy is needed to overcome the force of repulsion between the positively charged nuclei.
Explanation:
hope it helps
what makes your pulse? Explain
Answers
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:.
Answers
Answer: 3-nitropyridine
Explanation:
what is an environmental result from burning fossil fuels?
Answers
Answer:
The burning of fossil fuels contributes to the addition of greenhouse gases to the atmosphere. These gases trap thermal energy in the Earth's atmosphere.
help me please the subject is siecnce
Answers
Answer:
okay your question in science
What type of molecule is pentanal?
A. Aldehyde
O B. Ketone
O C. Alcohol
O D. Ester
Answers
Answer:
[tex] \huge \color{indigo} \boxed{a. \: aldehyde}[/tex]
Explanation:
Pentanal is considered to be a fatty aldehyde lipid molecule. These are compounds containing more than one aldehyde group. Pentanal is a very hydrophobic molecule, practically insoluble in water, and relatively neutral.
How many molecules are in 25.3g of C2H4O
Answers
Answer:
0.57 molecules
Explanation:
Part 1. After learning about the different parts and functions of a meroscope you will now reflect on the importance of ench part citeit significance of each parts by naming it and answering the guide question
Answers
Explanation:
after learning more about the position and would
What mass of water absorbs 6700 J of heat to raise the temperature from 283K to 318K?
Answers
Answer:
you did not give the specific heat like formula like it takes 1kj to raise 28grams of water by 10 grams
You have a sealed 2 liter flask that contains nothing but water and carbon dioxide. The flask is half-filled with liquid water, has a temperature of 25°c, and the overall pressure within the flask is 0. 1 atm. How many moles of co2 are in the flask? at this temperature, you may take the kh value for co2 as 0. 033 m / atm.
Answers
In this exercise we want to calculate the amount of moles, so this is going to be:
[tex](4.6)(10^{-3}) \ mols \ CO_2[/tex]
Knowing that Henry's law is given by:
[tex]C = KHP[/tex]
Where constants are given by:
C = Concentration KH = Henry's law constant = [tex]0.033 m/atm[/tex]P = partial pressure = [tex]0.07 atm[/tex]Before we can find the concentration of CO2 (and hence the moles of CO2), we first need to find its partial pressure. We look up the vapor pressure of water at 25º and find it to be 0.03 atm. Since the total pressure is equal to 0.1 atm, this mean the partial pressure of:
[tex]CO_2 = 0.1 \ atm - 0.03 \ atm = 0.07 \ atm[/tex]
Now using Henry's law, we find the concentration:
[tex]C = (0.033)*( 0.07) = (2.31)*(10^{-3})[/tex]
Converting to moles of CO2, we have:
[tex](2.31)*(10^{-3})*( 2) = (4.6)*(10^{-3})[/tex]
See more about concentration at https://brainly.com/question/3045247
the proton concentrations of three solutions at 25 °c are given. classify the solutions as acidic, basic, or neutral.
Answers
This problem is giving information about the proton concentrations of three solutions at 25 °C. Despite they are not numerically given, we can propose three scenarios to see how to approach the question.
Let the following solutions to come up:
[H⁺] = 2.63x10⁻³ M
[H⁺] = 1.00x10⁻⁷ M
[H⁺] = 4.511x10⁻⁹ M
The first step, will be the calculation of the pH for each solution via:
pH = -log([H⁺])
So that they turn out to be:
pH = -log(2.63x10⁻³ M) = 2.580
pH = -log(1.00x10⁻⁷ M) = 7.000
pH = -log(4.511x10⁻⁹ M) = 8.3457
In such a way, since acidic solutions have a pH below 7, neutral have a pH equal to 7 and basic have it above 7, we infer the first one is acidic, second one is neutral and third one is basic.
Thus, you can reproduce this methodology with the proton concentrations you are given.
Learn more:
https://brainly.com/question/23659500https://brainly.com/question/23428840What is an example of a polar molecule
Question 8 options:
A molecule that is made of ionic bonds like NaCl.
A molecule that is made of covalent bonds like sugar.
A molecule made of covalent bonds that has partial positive and partial negative charges in different areas of the atom.
A molecule made of ionic bonds that has strong positive and strong negative charges in different areas of the molecule.
Answers
Answer:
The two main classes of molecules are polar molecules and nonpolar molecules. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds.
Explanation:
so its c
An example of polar molecule is a molecule made of covalent bonds that has partial positive and partial negative charges in different areas of the atom.
What is covalent bond?
Covalent bond is defined as a type of bond which is formed by the mutual sharing of electrons to form electron pairs between the two atoms.These electron pairs are called as bonding pairs or shared pair of electrons.
Due to the sharing of valence electrons , the atoms are able to achieve a stable electronic configuration . Covalent bonding involves many types of interactions like σ bonding,π bonding ,metal-to-metal bonding ,etc.
Sigma bonds are the strongest covalent bonds while the pi bonds are weaker covalent bonds .Covalent bonds are affected by electronegativities of the atoms present in the molecules.Compounds having covalent bonds have lower melting points as compared to those with ionic bonds.
Learn more about covalent bond,here:
https://brainly.com/question/19382448
#SPJ2
which characteristic do valence electrons indicate about reactions between atoms?
Answers
Bond number
The characteristic of reactions that depends on valence electrons is the bond type.
In chemistry, a chemical bond could be;
Ionic
Covalent
The type of bond formed depends on the number of valence electrons present. When there are few valence electrons on an atom, it mostly forms ionic bonds.
When there are more electrons on an atoms, it mostly forms covalent bonds and the electrons between the atoms are shared.
How are covelant and ionic bonds different and what types of elements combine to form each?
Answers
Answer:
Ionic bonds result from the transfer of electrons from one atom to another
Explanation:
In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. The reaction components of covalent bonds are electrically neutral, whereas for ionic bonds they are both charged.Covalent bonds are formed between two non-metals, whereas ionic bonds are formed between a metal and non-metal.
Answer:
Covalent bonds are defined as sharing of electrons, ionic bonds are the result of transferred electrons;
Covalent bonds form between non-metals (elements) and ionic bonds form between metal and non-metals (ions)
¿Qué relación existe entre la teoría del octeto de Lewis con los enlaces iónicos y
covalentes?
Answers
Por definición de enlace iónico, covalente y regla del octeto, la relación existente entre la teoría del octeto de Lewis con los enlaces iónicos y covalentes es que dichos enlaces se producen con el objetivo de completar la última capa de electrones y adquirir estabilidad.
Enlace iónicoPor un lado, se produce un enlace iónico entre átomos metálicos y no metálicos, donde los electrones se transfieren completamente de un átomo a otro. Durante este proceso, un átomo pierde electrones y otro los gana, formando iones. Por lo general, el metal cede sus electrones formando un catión al elemento no metálico, que forma un anión.
Enlace covalentePor otro lado, el enlace covalente es el enlace químico entre átomos donde los electrones se comparten, formando una molécula. Se establecen enlaces covalentes entre elementos no metálicos. El par de electrones compartidos es común a los dos átomos y los mantiene unidos.
Regla del octetoEn ambos casos se cumple con la regla del octeto, que establece que los átomos de los elementos se enlazan unos a otros en el intento de completar su capa de valencia con ocho electrones. Es decir que los átomos van a tender a ceder o compartir electrones para completar ocho electrones en la capa de valencia mediante un enlace iónico, covalente o metálico.
En otras palabras, el objetivo es tener la configuración electrónica del gas noble más cercano, teniendo así la última capa de electrones completa y adquiriendo estabilidad.
En resumenEn resumen, la relación existente entre la teoría del octeto de Lewis con los enlaces iónicos y covalentes es que dichos enlaces se producen con el objetivo de completar la última capa de electrones y adquirir estabilidad.
Aprende más:
https://brainly.com/question/17100232?referrer=searchResultshttps://brainly.com/question/21960608?referrer=searchResults
Describe the two categories used to classify physical changes.
Answers
Answer:
Physical changes can be classified as reversible or irreversible. Melting is an example of a reversible physical change.
Explanation:
what is the periodic trend for electronegativity
Answers
Answer:
mark me brainlest
Explanation:
Electronegativity values generally increase from left to right across the periodic table. Electronegativities generally decrease from top to bottom of a group. The highest electronegativity value is for fluorine.
Answer:
"Electronegativity increases as you move from left to right across a period because the number of charges on the nucleus increases." (sstudy30 on Quizlet)
Explanation:
It explains itself haha!
Have a nice day!
I hope this is what you are looking for, but if not - comment! I will edit and update my answer accordingly. (ノ^∇^)
- Heather